 TRANSDERMAL DELIVERY
TRANSDERMAL DELIVERYTransdermal drug delivery as a route for systemic drug administration is currently one of the advancing areas in drug development research.
This is particularly true for the delivery of such drugs as opiate painkillers, birth control, nitroglycerin for the prophylaxis of angina, nicotine for smoking cessation therapy, testosterone for the correction of male hypogonadism.
The skin route for systemic drug delivery is especially attractive to formulators, as this integument is the most readily accessible organ of the body. Clinicians are increasingly recognising the advantages of controlled continuous intravenous infusion of drugs, such as the avoidance of hepatic metabolism and the maintenance of a constant therapeutic level in the body, that can be obtained by the use of the skin as a portal of drug delivery, without the risks and costs of intravenous therapy.
Drug-in-adhesive systems are characterised by the inclusion of the drug within the skin-contacting adhesive. The adhesive not only fulfils the adhesion to skin function, but serves as the formulation foundation containing drug and other excipients.
Transdermal patches are flexible pharmaceutical preparations of varying sizes containing both the drug and active ingredients, intended for application to unbroken skin to deliver an active ingredient to the systemic circulation. Such systems are designed to provide a delivery of active substance through intact skin with a constant systemic absorption rate.
Aside from transdermal delivery, many medical procedures require the attachment of a device to the skin surface, for example, catheters, electrodes and wound dressings.
Confidence in the adhesion and minimal skin disruption of all of these delivery methods are important as delivery of actives such as therapeutic agents may be necessary. There are subsequently crucial demands for adhesive products. They must stick to the application site only and there must be no unexpected adhesive failure, that is, they need to be reliable and durable. They must provide adequate adhesion and be gentle on removal (low peel force so that there is no skin stripping or pain). The development of the exact adhesive solution is made more difficult because the adherend is human skin, which has unlimited variability.
The performance properties of the adhesives can be evaluated using the well-established in vitro test methods for tack, peel adhesion and shear. However, modern equipment such as the Texture Analyser has been demonstrated to be an efficient analytical tool when comparative data, which do not meet the criteria required by traditional methods, need to be recorded on samples.
The use of extremely soft adhesives or elastic backings are examples of situations requiring the use of customised testing probes and testing procedures, which this technique allows. Each adhesive will have its own unique graphical representation or “fingerprint”. More insight into resulting performance properties can subsequently be obtained from this data than can be provided by a single data point test. The device manufacturer has a quick test to compare several adhesives and as a result conduct an efficient screening evaluation.
Measuring Transdermal patch adhesion
Skin adhesion and drug compatibility is one of the most critical performance characteristics of a transdermal system. Because drug delivery is directly proportional to the skin contact area, if a patch does not maintain proper skin adhesion, the drug will not be delivered at a constant rate.
Bioadhesives are now used in an extremely wide array of highly specific applications, each with specific environmental challenges. The best test methods are custom designed for each bioadhesive and application. For instance, instrumental adhesive testing should allow an operator to control the test conditions that both make and break the adhesive bonds in order to simulate an application. In order to repeatably measure the debonding process, one must precisely control the following elements during the bonding process: (i) the speed the probe travels until it touches the bioadhesive, (ii) the sensitivity by which the bioadhesive is detected, (iii) the speed at which the target force is applied, (iv) the force to be applied, (v) the duration of the applied force, (vi) the withdrawal speed, and (vii) the withdrawal distance. The bonding conditions significantly affect the debonding behaviour and therefore the measurement results.
During an adhesive test the probe (typically a domical probe or 1” diameter spherical probe for patch adhesion measurement as shown in Figure 5) descends to begin the bonding process and maintains the pre-determined compression force for the dwell time. During this stage the viscosity of the bioadhesive, the duration of the dwell and the chemistry of the materials will affect the strength of the created bond.
The probe then begins to withdraw and the adhesive elongates while stressing the just-created bonds. The sample is bonded adhesive side up to the test platform using double sided adhesive tape for successful measurement. Alternatively, for improved sample mounting and the quick transition to the next test site the Multiple Holed Plate in conjunction with an Adhesive Indexing Rig is employed. Information that can be obtained from the curves include area under the curve, peak force and distance to separation.
Temperature affects adhesion properties and so testing the adhesive strength of patch samples at a constant temperature (e.g. 35°C for skin) on a Peltier Plate (as shown in Figure 6), which provides a constant controlled testing platform to equilibrate the sample, thus providing a more accurate measurement.
Bioadhesion of Films
Topical delivery of certain drugs for the treatment of such conditions as dysplastic and neoplastic lesions has increased markedly in recent years. Whilst recommendations may be for a certain amount of cream to be applied per cm2 of lesional area for optimum results, clearly there is scope for considerable variation in the mass of applied cream per unit area of skin, driven presumably by the clinical preference and experience of those using the formulation.
Frequently, the cream is supplied on an outpatient basis. The variability in cream thickness, and hence applied dose, is likely to be even greater. The manufacturer may, for example, recommend that the cream should be left on the lesion for 6-10h. If the lesion is on an exposed area of skin, such as on the face or lower arm, then problems are likely.
However, if the lesion is situated in the lower female gynaecological tract, then cream retention for such long periods may prove difficult. Shear forces are high in this region, particularly in mobile patients and the cream may simply be sloughed away. It is conceivable then that variability in the dose of drug may lead to observed inconsistencies in the clinical response.
Similarly, percutaneous anaesthetic systems which traditionally are available as aqueous gel compositions, may prove impractical or inconvenient when, for example, large areas are to be treated, for example, prior to the harvesting of split skin grafts. A separate dressing to protect clothing is required and a patch presentation of the drug is then advantageous.
Novel skin patches in which moisture-activated adhesive (bioadhesive) polymer composition is combined with the moisture-activated drug release system to provide effective percutaneous anaesthesia are relatively simple to produce and are easily removed from the skin by peeling, leaving a negligible, water-soluble residue.
The design of drug-loaded bioadhesive patches is therefore an attempt to improve topical dosage systems. As the patch is of fixed dimension, variations in its thickness and the applied footprint do not occur. The topical dosage system then contains a defined drug loading available for release within a defined surface area.
Physical characterisation of the components of a bioadhesive patch for dose-controlled topical delivery of drugs is therefore necessary.
Measuring Bioadhesion of Topical Films
Hydrogel films can be cast and their bioadhesion assessed by attaching with double-sided adhesive tape to the underside of a cylinder probe and the exposed surface of neonate porcine skin pre-wetted with water. Adhesion is recorded as the force required to detach the sample from the surface of the excised skin after typically applying 2.5N for 30s and pulling away at 0.1mm/s. The distance to separation of a test film from the skin substrate, is also recorded to provide some measure of the cohesion within the film sample.
Alternatively for improved sample mounting and skin securing prior to testing a Flexible Substrate Clamp incorporating a multi-slot plate and a clamping fixture which work in conjunction with an Adhesive Indexing System (such as that shown in Figure 7). The sample is placed on the underside of the multi-slot plate, while the flexible substrate material is attached to the upper fixture. During the test, the arm of the Texture Analyser brings the attached material down into each slot so that it repeatedly contacts and withdraws from the sample. The maximum force required to withdraw from the sample is recorded, providing a measure of the adhesiveness.
Measuring Peel Strength of Patches/Wound Dressings
Dressings for wounds, burns and ulcers should actively support healing. The loss of skin integrity produces significant consequences for the individual. Hydroactive wound dressings retain exudate in the wound region or incorporate wound exudate by gel formation. They create a moist local environment for wound healing, which is experimentally and clinically characterised by accelerated reepithelialisation and angiogenesis as well as reduced wound pain and wound-infection rates.
Clinically relevant product groups of hydroactive wound dressings (hydrocolloids and hydropolymers, semipermeable films, and calcium alginates) are different in terms of chemical structure, physical properties and functional characteristics. Between the product groups, there are considerable differences with respect to inflammatory reactions at the wound base, absorption of exudate, occlusion properties, wound-edge adherence, adaptability to the wound shape and material integrity of wound dressings.
In the development of an integral patch device for percutaneous local anaesthesia, the properties of the dried intermediate film are essential in terms of the production, handling and clinical efficacy of the final product.
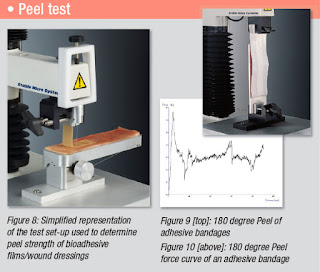
The peel strength of bi-laminar patch formulations can be investigated using neonate porcine skin secured to a sliding platform of a 90 Degree Peel Rig (as shown in Figure 8) using cyanoacrylate adhesive. Researchers have reported the use of the initial 1cm section of an elongated patch, measuring 1cm x 7cm, which is secured in the grips of a clamp, such that the remaining 6cm of length points vertically downwards. The clamp is brought to within 1cm of the porcine skin and water (10µl) placed on each 1cm2 to which the patch is adhered.
An area of the film measuring 1cm x 5cm is attached to the wetted skin using a force of 10Ncm2 applied for 30s. The clamp then moves upwards at a speed of 6mm/s. Simultaneously, the sliding section is free to move horizontally, such that the angle formed at the interface between the film and skin is maintained at approximately 90 degrees. Peel strength is recorded as the maximal force per unit length of the separating interface. This linear interface is formed at the point where tissue and bioadhesive matrix are pulled apart and represents the transverse width of the formulation (1cm).
Alternatively a 180 Degree Peel test can be carried out against a chosen rigid material (as shown in Figure 9/10).
Peel tests have been a popular test which have been traditionally performed. However, for accurate peel strength measurement a constant force application before removal is required and can be difficult to regulate. This can be facilitated by either a manual or automatic roller.
There is a Texture Analysis test for virtually any physical property. Contact Stable Micro Systems today to learn more about our full range of solutions.
For more information on how to measure texture, please visit the Texture Analysis Properties section on our website.
 The TA.XTplus texture analyser is part of a family of texture analysis instruments and equipment from Stable Micro Systems. An extensive portfolio of specialist attachments is
available to measure and analyse the textural properties of a huge range of
food products. Our technical experts
can also custom design instrument fixtures according to individual
specifications.
The TA.XTplus texture analyser is part of a family of texture analysis instruments and equipment from Stable Micro Systems. An extensive portfolio of specialist attachments is
available to measure and analyse the textural properties of a huge range of
food products. Our technical experts
can also custom design instrument fixtures according to individual
specifications.No-one understands texture analysis like we do!
To discuss your specific test requirements, click here...
 |  |  |



No comments:
Post a Comment