 INTRODUCTION
INTRODUCTIONDevelopments in transdermal, dermal and mucoadhesive drug delivery methods are paving the way to the future of systemic drug administration.
As skin is both the largest and most accessible organ in a human body, pharmaceutical manufacturers are keen to take advantage of it as a drug delivery platform.
The transdermal route helps to reduce the risks and costs of intravenous therapy that can be both traumatic and intrusive.
Transdermal controlled release (CR) systems usually combine a therapeutic component with an adhesive formulation that ensures a continuous delivery of the active ingredient through unbroken skin at a constant absorption rate. Skin adhesion and drug compatibility determine the efficacy of transdermal applications.
Adhesive properties are critical because the amount of medication delivered is directly proportional to the skin contact area. Transdermal systems need to cause minimum skin disruption, be easy to handle and be equally effective for the infinite variations in human skin. In vitro tests are commonly used to determine and compare tack, peel adhesion and shear characteristics of transdermal samples.
Automated texture analysis, facilitated through the use of a TA.XTplus texture analyser, for instance, allows such testing to be carried out in objectively controlled conditions that closely simulate real applications.
The following probes, fixtures and methods presented and outlined represent a non-exhaustive summary of the currently available and potentially invaluable solutions for the assessment of the physical properties of dermal, transdermal and mucoadhesive controlled release products.
DERMAL DELIVERY
Creams, lotions and topical gels
Target sites for dermal products such as creams, lotions and gels are the dermis, epidermis, hair follicle, etc. However the big challenge regarding dermal use is controlling the amounts used by patients. Different patients use different amounts of the product which is difficult to control and the area of skin covered is also a variable. Since the product is designed to achieve zero-bioavailability there are very few side effects, barring skin irritation, and this is the biggest advantage of dermal delivery. The treatment is local and does not expose the whole body to the drug. Other challenges deal with stability issues. Since emulsions are used, ensuring that phase separation does not take place between the two immiscible components is important.
Pharmaceutically, water soluble cellulose polymers have found widespread applications, e.g. in the formulation of solid dosage forms, aqueous disperse systems as viscosity enhancing agents and in products for topical application. In the development of topical dosage forms, several desirable attributes that contribute to the ultimate patient acceptability and clinical efficacy of the product may be defined.
These include optimal mechanical properties (e.g. ease of removal of the product from the container, good spreadability on the substrate, e.g. skin, mucous membranes), good bioadhesion (to ensure retention at the site of application), acceptable viscosity, drug release and drug absorption. However, products designed for topical administration will be subjected to shearing forces, e.g. chewing, breathing, swallowing, talking, flexing processes of skin and it is therefore important to examine the effects of such actions on the product rheology, and hence, on their clinical performance.
Using Texture Profile Analysis (TPA)
A rapid, straightforward analytical technique, texture profile analysis (TPA) has been found useful by researchers at Queen’s University Belfast to apply to the mechanical characterisation of pharmaceutical gels and semi-solid systems. In this type of test, a cylinder probe is twice depressed into a contained sample (as shown in Figure 1) to a defined depth and at a defined rate, allowing a delay period between successive compressions.
From the resultant force-time plot (Figure 2), the following mechanical parameters may be described:
1: Hardness (force required to attain a given deformation)
2: Adhesiveness (the work necessary to overcome the attractive forces between the surface of the sample and the surface of the probe)
3: Compressibility (the work required to deform the product during the first compression of the probe).
Whilst this test was originally developed for the assessment of self-supporting products by compression, these mechanical properties have been directly correlated with sensory properties in vivo, e.g. removal of the product from the container, application characteristics of the product, and are therefore directly applicable to the design of topical pharmaceutical preparations.
Ideally, formulations designed for application to, for example, the periodontal pocket should exhibit controlled release of drug within, and ease of delivery of the formulation into and good retention within the periodontal pocket.
Optimisation of the mechanical and viscoelastic properties of such formulations is important as these properties will affect their clinical efficacy. Formulations designed for the periodontal pocket should ideally possess low hardness and compressibility, yet high adhesiveness.
These properties will ensure ease of administration of the formulations into the pocket, whereas, high values of adhesiveness, a property related to bioadhesion, indicates good retention within the pocket. The effects of each polymeric component on hardness and compressibility may be related to their effects on product viscosity/ consistency, a measure of resistance to product compression.
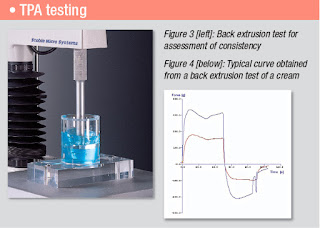
An equally successful approach is to perform a Back Extrusion test (Figure 3) using a disc to push into the viscous sample and measure the resistance to flow around the disc and then the resistance to withdrawal from the sample. Such a test gives a typical graph (Figure 4) which allows the measurement of maximum positive and negative forces and both positive and negative areas (‘works of extrusion’) all giving a comprehensive set of consistency parameters.
There is a Texture Analysis test for virtually any physical property. Contact Stable Micro Systems today to learn more about our full range of solutions.
For more information on how to measure texture, please visit the Texture Analysis Properties section on our website.
 The TA.XTplus texture analyser is part of a family of texture analysis instruments and equipment from Stable Micro Systems. An extensive portfolio of specialist attachments is
available to measure and analyse the textural properties of a huge range of
food products. Our technical experts
can also custom design instrument fixtures according to individual
specifications.
The TA.XTplus texture analyser is part of a family of texture analysis instruments and equipment from Stable Micro Systems. An extensive portfolio of specialist attachments is
available to measure and analyse the textural properties of a huge range of
food products. Our technical experts
can also custom design instrument fixtures according to individual
specifications.No-one understands texture analysis like we do!
To discuss your specific test requirements, click here...
|





No comments:
Post a Comment