Mucoadhesion is the process by which a drug delivery device is designed to stick to a part of the gut or other mucosae (buccal cavity, nasal, rectal and vaginal), thus delivering drug to a precise site in the body for an extended period.
This gives more effective treatment of some diseases and can also protect drugs from some of the harsh conditions in the body. Mucoadhesive drug delivery systems are used to treat several conditions in the mouth and have been investigated as treatments for stomach ulcers and cancer. The majority of infections affecting man and animals take place or start in mucous membranes. The ability to retain pharmacologically-active agents for extended periods of time on any mucosal epithelium, including those of the nose, mouth, rectum or vagina confers several potential therapeutic advantages.
Whilst mucosal delivery is the smallest market for drug delivery systems, as it has the lowest patient acceptance, delivery across the mucosa of the mouth and the nose are gaining a market presence, providing rapid onset in an acceptable form. The possibility of delivering genes and vaccines by this method is igniting excitement in the industry, making the future look brighter. In terms of nasal delivery, rapid onset and instant relief, especially in the area of pain management, is one big advantage of this delivery system. Very small doses are highly efficient through this route, hence only low concentrations are required. Patient comfort and compliance and high costs are two barriers to be overcome. The nose is the best place to deliver virtually any drug but this route can cause irritation, and hence patient discomfort.
Mucoadhesion of Films
The delivery of drugs to the oral cavity can pose a number of problems. Recently there has been a growing interest in using bioadhesive films in the buccal cavity for systemic or local drug delivery. Buccal drug delivery films could be a better option than buccal tablets especially with regard to ease of use and the flexibility of the delivery system. Films can give a protective layer across ulcers and thus reduce pain. Buccal films also give the possibility of local treatment of buccal diseases.
Films are matrix systems, where a drug is dispersed in polymer and release of the drug is due to diffusion of the drug out of the system or because of the disintegration of the system. It is simple to control the size and shape of films according to the area intended for treatment and application to the oral mucosa is easy and usually straightforward for the patient. Buccal mucosal drug delivery has several other advantages, such as lower first pass effect and the possibilities of using lower doses. Sodium alginate is a biopolymer that has exhibited good bioadhesive properties and has been used as an excipient in various dosage forms. As with all potential excipients and drug delivery systems their suitability needs to be evaluated in many ways.
Mucoadhesion of Powders
Whilst tablets have more commonly been assessed for bioadhesion, individual microspheres and powder have also been investigated. Powders have been suggested as providing a simple, rapid method of measuring the adhesive properties of a material. Normally, the concept of enhanced bioadhesion is discussed in the preparation of controlled release formulations and, in these cases, it is obviously important to use the actual product for the measurement of bioadhesion.
However, such concepts could also be applicable to instant-release formulations, such as tablets for sublingual administration. In these formulations, after initial rapid disintegration, the tablet subunits formed should preferably adhere for a limited period to the sublingual mucosa, so as to avoid swallowing and systemic uptake from the intestine. For such specific application, a non-disintegrating tablet form will obviously not be a suitable specimen for bioadhesion testing. The use of powder particles would better reflect the adhesion of subunits or particles to the mucosa after tablet disintegration.
Mucoadhesion of Gastrospheres
In the case of absorption of certain drugs limited to a narrow specific site, such as the duodenum, decreased bioavailability of the drug and the subsequent requirement for frequent dosing may necessitate the drug delivery system to be modified to retard gastrointestinal tract transit time in order to increase bioavailability. A multi-particulate gastrofloatable and gastroadhesive drug delivery system, termed gastrospheres, presents a delivery system within the gastric region for at least 12 hours, delivering small quantities of drug over an extended period of time, thus, resulting in a constant flow of drug being in contact with the specific site of absorption over the duration of drug therapy.
Mucoadhesion of Polymers
The use of solid mucoadhesive dosage forms (films, patches, tablets, coated micro spheres, etc) dominates in tensile testing, since it is known that the adhesiveness decreases rapidly when certain levels of hydration are achieved. However, there is also a need to test partially hydrated polymers, at least in order to investigate semi-solid mucoadhesives. A further, more general consideration is that gel formation is an inevitable step in mucoadhesion, hence there is a need to study the processes associated with polymer-mucin gel interactions.
Measuring Mucoadhesion
The TA.XTplus Texture Analyser has emerged as a useful tool for measuring bioadhesion bond strength. The determination of the adhesive properties of pharmaceutical dosage forms is important in their development and several methods have been developed for these measurements. Tests of solid dosage forms, semi-solids such as ointments and gels and even systems which solidify on contact with the target organ can be performed using alternative measuring probes.
The measurement of adhesive properties has already been reviewed for transdermal adhesive products and the same adhesive test guidelines and curve analysis techniques apply for the measurement of mucoadhesion. Sample preparation and testing method techniques, however, vary depending upon the nature of the sample. The following points aim to provide an understanding of the alternative sample preparation methods, probe options and necessary method adjustments to assist in the design of custom tests, particularly for mucoadhesion testing.
Developed at the University of Strathclyde, and since adopted by a number of groups in Britain and Europe, the Mucoadhesion Rig offers a number of advantages over systems previously used for the assessment of mucoadhesion. Where conditions close to those found in vivo are required, the Mucoadhesion Rig provides the ability to set-up the tissue samples in a vessel of temperature regulated gastric fluid and lower a probe with the attached solid or semi-solid dosage form onto the tissue (as shown in Figure 11).
Sample Preparation Alternatives
Porcine mucosa is the membrane typically used for bioadhesive measurements. As the tissue itself is often inconsistent, the sample preparation does demand consistent harvesting, trimming, storing and environmental conditioning of the sample so as not to pose a barrier to the experimentation required to optimise the test methods. Artificial membranes have also been used as these simplify the sample preparation difficulties when using biological membrane.
Several published papers examine the bond of bioadhesives against glass or metal cylinders.
Even though bioadhesives can adhere differently to different materials than to tissue, often the relative changes in the adhesiveness correlate well against expectations (due to changes in bioadhesive ingredients). For this reason solid materials (stainless steel, aluminium, acrylic, glass, etc) may be acceptable to examine relative adhesive behaviours, with the added benefit that sample handling protocols are much simpler.
Where the mucosa is held in ambient conditions without suspension in, for example, gastric fluid, a fixed volume of buffer is generally pipetted onto the mucosa to standardise the hydration prior to testing.
The most common probe sizes and dimensions for bioadhesive testing are acrylic or similar cylinders with a diameter of 7-10 mm. Solid materials such as tablets and films (of standard section size) are usually attached to the underside of the upper testing probe using cyanoacrylate adhesive or double-sided tape.
For the assessment of powders, the application of the powder can be performed by immersing the probe (with adhesive tape attached to the underside) into a powder bed and thereafter gently shaking the probe to remove any excess, to achieve a monolayer of particles. When selecting a double-sided tape, the thinnest and stiffest tape possible should be chosen since the material must not be allowed to flex or loosen during debonding.
Samples of gastrospheres have been successfully tested by previously immersing in simulated gastric fluid for predetermined time intervals, covering both the probe and the test platform with simulated gastric membrane and measuring the bioadhesion (the force of detachment) of samples after applying a force of 2N.
Researchers Skulason, Kristmundsdottir and Holbrook in the Faculty of Pharmaceutical Sciences at the University of Iceland more recently presented a technique for evaluation of the adhesion of hydrogel compositions. The use of bioadhesive polymers in the formulation of dosage forms for mucosal drug delivery is receiving increasing attention and whilst the mucoadhesion of solid dosage forms using the Mucoadhesion Rig has been frequently employed by researchers in this field, the measurement of hydrogel mucoadhesion had not previously been thoroughly investigated.
 Mucin discs have been
reported to have been prepared and attached to the underside of a
cylinder probe using double sided adhesive tape and then lowered onto
the surface of the gel formulation applying a downward force for a
predefined time and measuring the force required to detach the mucin
disc from the surface of each formulation. However, a particular
advantage of the Mucoadhesion Rig is the ability to test tissue samples
in the conditions in which they are normally found.
Mucin discs have been
reported to have been prepared and attached to the underside of a
cylinder probe using double sided adhesive tape and then lowered onto
the surface of the gel formulation applying a downward force for a
predefined time and measuring the force required to detach the mucin
disc from the surface of each formulation. However, a particular
advantage of the Mucoadhesion Rig is the ability to test tissue samples
in the conditions in which they are normally found. An alternative method is now available which enables attachment of gels to a probe. The Gel Mucoadhesion Probe (Figure 12) consists of an inverted cone shape at its end which has machined concentric grooves. These grooves encourage the attachment of a controlled volume of hydrogel sample to the probe surface area.
For constant gel volume application the use of a syringe is recommended. A PTFE collar is supplied to support larger volumes of hydrogel loading which is removed when the gel is set.
Analysis of Adhesion Test Results
As previously explained, during an adhesive test the probe (usually a cylinder probe for mucoadhesion applications) descends to begin the bonding process and maintains the pre-determined compression force for the dwell time.
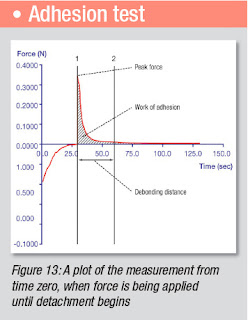
After this time, the probe withdraws from the mucosal tissue and the adhesive properties are measured (see Figure 13 for typical graph). The force needed to detach the hydrogel is recorded as a function of elongation and both maximum strength and area under the force/time curve is usually obtained. The results can be converted into work of adhesion (mJ/cm2) and then represented as a mean value with standard deviation.
Once an adhesive test is generated, analysing the debonding behaviour can be much more complex than simply tracking peak force which has commonly been collected in the past.
Whilst peak forces for different products can be similar, the debonding behaviour shown by the calculation of parameters from other regions of the curve, such as work of adhesion and debonding distance, can be very different.
For the measurement of tensile stress (N/cm2) the detachment force is divided by the surface area of the tablet/gel sample or probe surface area (for films and powders). Figure 13 is an annotated adhesive curve.
A mucoadhesive which must hold a drug against throat linings, where food, liquid and muscles are capable of dislodging it, must hold under very aggressive conditions. Area of work is a much better measure of its adhesive capability than peak force, since the product must withstand the dislodging forces. Few real forces in many mucoadhesive situations are sharp enough to generate momentarily high peak forces.
For most adhesive plots, if the distance after the peak force is great (relative to the distance to the peak – and without regard to the areas of work) then the product has likely failed internally, indicating poor cohesion. The same behaviour tends to be true for the area of work after the peak.
Thus low ratios of either (post distance)/(pre distance) or (post area)/(pre area) indicate strong relative cohesion; and high ratios would indicate poor cohesion. The stringiness or ‘legs’ of bioadhesives are quantifiable by the displacement distances to the peak force and to the final debonding.
The adhesive bond of a highly-cohesive bioadhesive (e.g. a transdermal patch) is typically weaker than its adhesiveness to the probe, so separation from the probe can be immediate. Very cohesive products typically leave no residue on the probe since the failure is at the adhesive surface and the force drop-off is crisp.
A few complex polymers will experience early adhesive and cohesive failures, followed by increasing resistance due to the changing and strengthening adhesive filaments or to strain hardening behaviourIt is desirable that a mucoadhesive be extremely cohesive so that it can stay intact as the body attempts to void it.
Poorly cohesive bioadhesives (for example, medicinal eye-drops and mucoadhesives) must nevertheless be sufficiently strong to stay in place during the initial application. When poorly-cohesive bioadhesive debond, they tend to deform in an ‘hourglass’ shape before failing cohesively.
These products typically leave residue on the probe surface since the failure is entirely within the adhesive, and the force drop-off tends to be gradual. For such poorly cohesive products higher areas of work indicates that the product has remained intact, perhaps sufficiently for a smaller drug dose to be adequate for the medical challenge.
THE FUTURE OF CONTROLLED RELEASE PRODUCTS
The stability of a pharmaceutical product is paramount to the consumer’s acceptance of it, as well as to its subsequent efficacy and safety. Improving excipients and other ingredients can make drug delivery much more effective at lower costs, but those benefits can be mitigated when quality control problems arise during manufacturing.
It is vital, therefore, that manufacturers scientifically assess any potential changes in the structure or character of their products throughout formulation, processing and distribution. That is where texture analysis plays a role in providing real-time information on the effects on physical quality of active ingredients, inactive ingredients and finished products.
The desired goal is to design and develop processes that can consistently ensure a predefined quality at the end of the manufacturing process. Texture analysis instrumentation has enabled manufacturers to do just this, offering targeted, repeatable testing that produces actionable data.
As the pharmaceutical industry innovates, so materials analysis evolves, developing and adapting to provide new instruments and methodologies for emerging requirements.
There is a Texture Analysis test for virtually any physical property. Contact Stable Micro Systems today to learn more about our full range of solutions.
For more information on how to measure texture, please visit the Texture Analysis Properties section on our website.
 The TA.XTplus texture analyser is part of a family of texture analysis instruments and equipment from Stable Micro Systems. An extensive portfolio of specialist attachments is
available to measure and analyse the textural properties of a huge range of
food products. Our technical experts
can also custom design instrument fixtures according to individual
specifications.
The TA.XTplus texture analyser is part of a family of texture analysis instruments and equipment from Stable Micro Systems. An extensive portfolio of specialist attachments is
available to measure and analyse the textural properties of a huge range of
food products. Our technical experts
can also custom design instrument fixtures according to individual
specifications.No-one understands texture analysis like we do!
To discuss your specific test requirements, click here...
 |  |  |


No comments:
Post a Comment